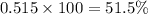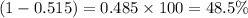The average molecular weight for element x is 59.97 g/mol. there are two known isotopes of element x, one weighing 59 g/mol, and the other weighing 61 g/mol. what is the relative abundance of each?
possible answers:
50% x-59, 50% x-61
75% x-59, 25% x-61
63% x-59, 37% x-61
51.5% x-59, 48.5% x-61
48.5% x-59, 51.5% x-61

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 2

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
The average molecular weight for element x is 59.97 g/mol. there are two known isotopes of element x...
Questions


Mathematics, 21.12.2020 17:30


English, 21.12.2020 17:30

Mathematics, 21.12.2020 17:30


Mathematics, 21.12.2020 17:40

Mathematics, 21.12.2020 17:40


History, 21.12.2020 17:40


Engineering, 21.12.2020 17:40



Mathematics, 21.12.2020 17:40


Computers and Technology, 21.12.2020 17:40



Physics, 21.12.2020 17:40

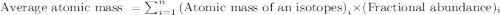 .....(1)
.....(1)![59.97 g/mol=[(59 g/mol\times x)+(61 g/mol\times (1-x))]\\\\x=0.515](/tpl/images/0202/9296/4c349.png)