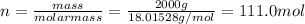Chemistry, 27.08.2019 17:10 Jackiebear4593
Assuming the mass of the ice in the beaker was 2000 g (2 kg), calculate the amount of heat that must have been added to convert the ice to water (assume that the entire 2000 g was ice at the start of melting, and the entire 2000 g was water at the end of the melting). assume that the change took place entirely at 0 °c. the heat of fusion (dhfus) for h2o is 6.12 kj/mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2
You know the right answer?
Assuming the mass of the ice in the beaker was 2000 g (2 kg), calculate the amount of heat that must...
Questions

Mathematics, 30.10.2020 19:30


Mathematics, 30.10.2020 19:30



Mathematics, 30.10.2020 19:30

Biology, 30.10.2020 19:30

History, 30.10.2020 19:30


Mathematics, 30.10.2020 19:30

English, 30.10.2020 19:30


Mathematics, 30.10.2020 19:30

Social Studies, 30.10.2020 19:30


History, 30.10.2020 19:30

Mathematics, 30.10.2020 19:30

Mathematics, 30.10.2020 19:30

Computers and Technology, 30.10.2020 19:30