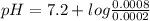Chemistry, 27.08.2019 17:10 desikayla2013
Ph indicator. a dye that is an acid and appears as different colors in its protonated and deprotonated forms can be used as a ph indicator. suppose that you have a 0.001 m solution of a dye with a p k a of 7.2. from the color, the concentration of the protonated form is found to be 0.0002 m. assume that the remainder of the dye is in the deprotonated form. what is the ph of the solution?

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 23:00
The wave shown on the electromagnetic spectrum disturb the medium it passes through a)different frequency. b)the same frequency .
Answers: 2

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1
You know the right answer?
Ph indicator. a dye that is an acid and appears as different colors in its protonated and deprotonat...
Questions


Mathematics, 20.04.2021 18:20

Health, 20.04.2021 18:20


Biology, 20.04.2021 18:20

Mathematics, 20.04.2021 18:20



History, 20.04.2021 18:20


Biology, 20.04.2021 18:20



Mathematics, 20.04.2021 18:20


History, 20.04.2021 18:20

Physics, 20.04.2021 18:20

Mathematics, 20.04.2021 18:20

Physics, 20.04.2021 18:20

![pH = p_{ka} +log\frac{[Salt]}{[Acid]}](/tpl/images/0202/9224/babd2.png)