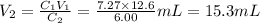Chemistry, 27.08.2019 03:10 amulets7017
Achemist must dilute 12.6ml of 7.27 m aqueous sodium nitrate (nano3) solution until the concentration falls to 6.00 m. he'll do this by adding distilled water to the solution until it reaches a certain final volume. calculate this final volume, in milliliters. be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
Achemist must dilute 12.6ml of 7.27 m aqueous sodium nitrate (nano3) solution until the concentratio...
Questions


Mathematics, 24.11.2020 21:20

Mathematics, 24.11.2020 21:20

Social Studies, 24.11.2020 21:20

History, 24.11.2020 21:20



English, 24.11.2020 21:20


Physics, 24.11.2020 21:20

Mathematics, 24.11.2020 21:20



Mathematics, 24.11.2020 21:20


Mathematics, 24.11.2020 21:20



Mathematics, 24.11.2020 21:20

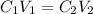
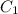 is initial concentration,
is initial concentration, 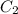 is the final concentration,
is the final concentration, 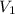 is the initial volume and
is the initial volume and 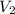 is the final volume.
is the final volume.