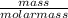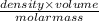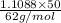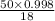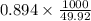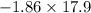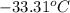Chemistry, 27.08.2019 02:30 marissa2367
The coolant in automobiles is often a 50/50 % by volume mixture of ethylene glycol, c2h6o2, and water. at 20°c, the density of ethylene glycol is 1.1088 g/ml and the density of water is 0.9982 g/ml. what is the expected freezing point of a 50/50(v/v)% ethylene glycol/water solution? kf = 1.86°c/m for water.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write two balanced equations 1. dissolving of solid sodium hydroxide in water 2. the reaction of sodium hydroxide solution with hydrochloric acid
Answers: 1

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
The coolant in automobiles is often a 50/50 % by volume mixture of ethylene glycol, c2h6o2, and wate...
Questions




Biology, 07.04.2020 04:51







History, 07.04.2020 04:51

Biology, 07.04.2020 04:52

Health, 07.04.2020 04:52




Mathematics, 07.04.2020 04:52


Social Studies, 07.04.2020 04:52

Mathematics, 07.04.2020 04:52