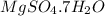Chemistry, 26.08.2019 22:10 cecelia090
Epsom salts is a hydrated ionic compound with the following formula: mgso4⋅x h2o. a 4.93-g sample of epsom salts is heated to drive off the water of hydration. the mass of the sample after complete dehydration is 2.41 g. find the number of waters of hydration (x) in epsom salts.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2


You know the right answer?
Epsom salts is a hydrated ionic compound with the following formula: mgso4⋅x h2o. a 4.93-g sample o...
Questions

Social Studies, 18.08.2019 21:30


Biology, 18.08.2019 21:30

English, 18.08.2019 21:30

Mathematics, 18.08.2019 21:30

Mathematics, 18.08.2019 21:30


Mathematics, 18.08.2019 21:30


Mathematics, 18.08.2019 21:30


History, 18.08.2019 21:30





Social Studies, 18.08.2019 21:30



Biology, 18.08.2019 21:30

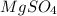 = 120 g/mol
= 120 g/mol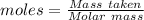
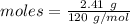
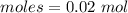
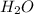 = 18 g/mol
= 18 g/mol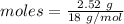
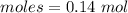
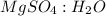 = 0.02 : 0.14 = 1 : 7
= 0.02 : 0.14 = 1 : 7