
Chemistry, 26.08.2019 20:30 BandNerd922
Concentrated hydrogen peroxide solutions are explosively decomposed by traces of transition metal ions (such as mn or fe): 2h2o2(aq) > 2h2o(l) + o2(g)
what volume of pure o2(g), collected at 27c and 746 torr, would be generated by decomposition of 125 g of a 50.0% by mass hydrogen peroxide solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2



Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1
You know the right answer?
Concentrated hydrogen peroxide solutions are explosively decomposed by traces of transition metal io...
Questions

Mathematics, 30.04.2021 21:50

Mathematics, 30.04.2021 21:50

Chemistry, 30.04.2021 21:50


Mathematics, 30.04.2021 21:50






Mathematics, 30.04.2021 21:50



Mathematics, 30.04.2021 21:50

Mathematics, 30.04.2021 21:50

Mathematics, 30.04.2021 21:50




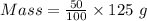
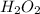 = 34 g/mol
= 34 g/mol
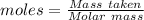
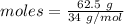
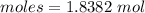
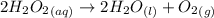
![\frac {1}{2}\times 1.8382 mole of oxygen gas. Moles of oxygen gas produced = 0.9191 molGiven: Pressure = 746 torr The conversion of P(torr) to P(atm) is shown below: [tex]P(torr)=\frac {1}{760}\times P(atm)](/tpl/images/0200/3064/bae8f.png)



