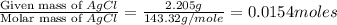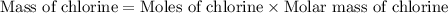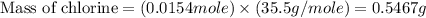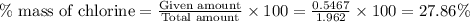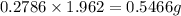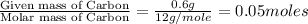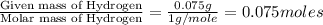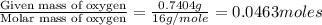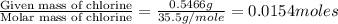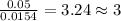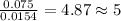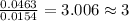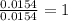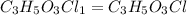Chemistry, 26.08.2019 19:30 06laurenelizabeth
Acompound contains c, h, cl and o. combustion of 1.962 g of the compound gave 2.200 g co2 and 0.676 g h2 o. in a separate analysis 1.208 g of the compound was converted into 2.205 g agcl. the approximate molecular weight is 160. what is the empirical formula?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1


Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1
You know the right answer?
Acompound contains c, h, cl and o. combustion of 1.962 g of the compound gave 2.200 g co2 and 0.676...
Questions



Chemistry, 29.07.2019 20:10



Chemistry, 29.07.2019 20:10



Mathematics, 29.07.2019 20:10

History, 29.07.2019 20:10










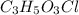
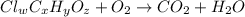
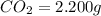
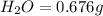
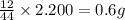 of carbon will be contained.
of carbon will be contained.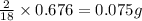 of hydrogen will be contained.
of hydrogen will be contained.