
Chemistry, 25.08.2019 21:10 Andrebutrus
***30
if 25.0 ml of a 0.100 m aqueous sodium hydroxide is mixed with 25.0 ml of a 0.100 m aqueous hydrochloric acid in a calorimeter at an initial temperature of 23.0 degrees celsius, what is the enthalpy change of this reaction if the final temperature reached in the calorimeter is 25.5 degrees celsius?
naoh + hcl yields nacl + h2o

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1


Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2
You know the right answer?
***30
if 25.0 ml of a 0.100 m aqueous sodium hydroxide is mixed with 25.0 ml of a 0.100 m aqu...
if 25.0 ml of a 0.100 m aqueous sodium hydroxide is mixed with 25.0 ml of a 0.100 m aqu...
Questions







Mathematics, 07.07.2020 14:01


Computers and Technology, 07.07.2020 14:01

Mathematics, 07.07.2020 14:01


English, 07.07.2020 14:01

Geography, 07.07.2020 14:01



Biology, 07.07.2020 14:01

English, 07.07.2020 14:01

SAT, 07.07.2020 14:01

English, 07.07.2020 14:01

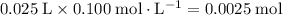 of both NaOH and HCl are available. As a result, 0.0025 moles of the reaction would have taken place.
of both NaOH and HCl are available. As a result, 0.0025 moles of the reaction would have taken place.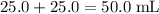 .
.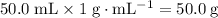 .
.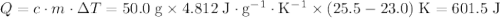
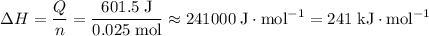 .
.


