Chemistry, 24.08.2019 04:30 carlosleblanc26
In the extraction of aluminium from bauxite ore, several steps are involved. in one of these, sodium hydroxide (naoh) reacts with aluminium oxide (al2o3) to produce sodium aluminate (naalo2) and water. write down a balanced chemical equation showing this reaction. explain how you can test that your equation is balanced.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which feature do highland climates have that lower elevation areas do not?
Answers: 1

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2
You know the right answer?
In the extraction of aluminium from bauxite ore, several steps are involved. in one of these, sodium...
Questions


Mathematics, 10.02.2021 06:00

Mathematics, 10.02.2021 06:00

Mathematics, 10.02.2021 06:00

Biology, 10.02.2021 06:00

Mathematics, 10.02.2021 06:00

Mathematics, 10.02.2021 06:00

Mathematics, 10.02.2021 06:00


Health, 10.02.2021 06:00


Mathematics, 10.02.2021 06:00





Mathematics, 10.02.2021 06:00


Chemistry, 10.02.2021 06:00

Arts, 10.02.2021 06:00

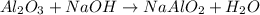
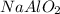 by 2 on product side.
by 2 on product side.