
Chemistry, 23.08.2019 04:30 elizabethburkha
An electrochemical cell at 25°c is composed of pure copper and pure lead solutions immersed in their respective ionis. for a 0.6 m concentration of cu2+, the lead electrode is oxidized yielding potential of 0.507 v. a cell a) calculate the concentration of pb2+ b) suppose the lead electrode is reduced, in that case what would be the concentration of pb2 what does this answer tell you?

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2
You know the right answer?
An electrochemical cell at 25°c is composed of pure copper and pure lead solutions immersed in their...
Questions



Computers and Technology, 30.04.2021 16:10

Mathematics, 30.04.2021 16:10


Mathematics, 30.04.2021 16:10


Physics, 30.04.2021 16:10

Mathematics, 30.04.2021 16:10

Social Studies, 30.04.2021 16:10



Mathematics, 30.04.2021 16:10





History, 30.04.2021 16:10

History, 30.04.2021 16:10

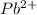 is, 0.0337 M
is, 0.0337 M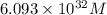
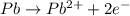
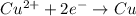
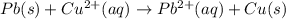
![E^o_{[Pb^{2+}/Pb]}=-0.13V](/tpl/images/0189/9678/d356c.png)
![E^o_{[Cu^{2+}/Cu]}=+0.34V](/tpl/images/0189/9678/0d165.png)
![E^o=E^o_{[Cu^{2+}/Cu]}-E^o_{[Pb^{2+}/Pb]}](/tpl/images/0189/9678/2597c.png)
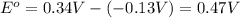
![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Pb^{2+}]}{[Cu^{2+}]}](/tpl/images/0189/9678/2e714.png)
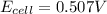
![0.507=0.47-\frac{0.0592}{2}\log \frac{[Pb^{2+}]}{(0.6)}](/tpl/images/0189/9678/33110.png)
![[Pb^{2+}]=0.0337M](/tpl/images/0189/9678/42065.png)
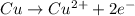
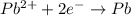
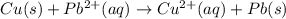
![E^o=E^o_{[Pb^{2+}/Pb]}-E^o_{[Cu^{2+}/Cu]}](/tpl/images/0189/9678/264d0.png)
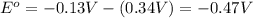
![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Cu^{2+}]}{[Pb^{2+}]}](/tpl/images/0189/9678/9bec7.png)
![0.507=-0.47-\frac{0.0592}{2}\log \frac{(0.6)}{[Pb^{2+}]}](/tpl/images/0189/9678/15aa8.png)
![[Pb^{2+}]=6.093\times 10^{32}M](/tpl/images/0189/9678/e8ea8.png)


