

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2


Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1
You know the right answer?
Calculate the change in the standard entropy of the system, delta s degree for the synthesis of ammo...
Questions

Mathematics, 04.05.2021 16:00


Chemistry, 04.05.2021 16:00

Mathematics, 04.05.2021 16:00




Mathematics, 04.05.2021 16:00

Mathematics, 04.05.2021 16:00



Mathematics, 04.05.2021 16:00

Computers and Technology, 04.05.2021 16:00

Mathematics, 04.05.2021 16:00


World Languages, 04.05.2021 16:00


Mathematics, 04.05.2021 16:00

Mathematics, 04.05.2021 16:00

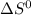 for the reaction is -198.762 J/K
for the reaction is -198.762 J/K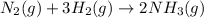
![\Delta S^{0}=[2moles\times S^{0}(NH_{3})_{g}]-[1mole\times S^{0}(N_{2})_{g}]-[3\times S^{0}(H_{2})_{g}]](/tpl/images/0189/5076/f0e35.png)
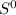 represents standard entropy.
represents standard entropy.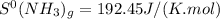 ,
, 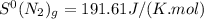 and
and 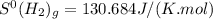
![\Delta S^{0}=[2\times 192.45]-[1\times 191.61]-[3\times 130.684]J/K=-198.762J/K](/tpl/images/0189/5076/4d0f0.png)


