
Chemistry, 22.08.2019 22:30 emmaraeschool
The equilibrium constant kc for the reaction pcl3(g) + cl2(g) ⇌ pcl5(g) is 49 at 230°c. if 0.70 mol of pcl3 is added to 0.70 mol of cl2 in a 1.00-l reaction vessel at 230°c, what is the concentration of pcl3 when equilibrium has been established? a) 0.049 mb) 0.11 mc) 0.59 md) 0.30 me) 0.83 m

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is mostly likely why many scientists reject the cold fusion theory
Answers: 1

Chemistry, 21.06.2019 18:00
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2
You know the right answer?
The equilibrium constant kc for the reaction pcl3(g) + cl2(g) ⇌ pcl5(g) is 49 at 230°c. if 0.70 mol...
Questions


Mathematics, 30.03.2020 21:23



Mathematics, 30.03.2020 21:23

History, 30.03.2020 21:23



Biology, 30.03.2020 21:23


Mathematics, 30.03.2020 21:23


History, 30.03.2020 21:23

Mathematics, 30.03.2020 21:23


History, 30.03.2020 21:23

Mathematics, 30.03.2020 21:23



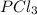 and
and 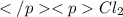 .
.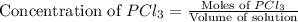
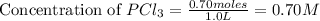
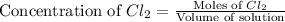
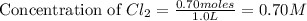
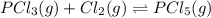
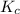 will be,
will be,![K_c=\frac{[PCl_5]}{[PCl_3][Cl_2]}](/tpl/images/0189/1195/4c8d0.png)
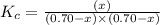
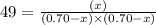
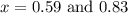
 at equilibrium = (0.70-x) = (0.70-0.59) = 0.11 M
at equilibrium = (0.70-x) = (0.70-0.59) = 0.11 M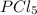 at equilibrium = x = 0.59 M
at equilibrium = x = 0.59 M

