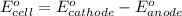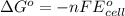Chemistry, 22.08.2019 21:30 trosclairozlynn02
Using the following standard reduction potentials, fe3+(aq) + e- → fe2+(aq) e° = +0.77 v ni2+(aq) + 2 e- → ni(s) e° = -0.23 v calculate the standard cell potential for the galvanic cell reaction given below, and determine whether or not this reaction is spontaneous under standard conditions. ni2+(aq) + 2 fe2+(aq) → 2 fe3+(aq) + ni(s)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1
You know the right answer?
Using the following standard reduction potentials, fe3+(aq) + e- → fe2+(aq) e° = +0.77 v ni2+(aq) +...
Questions



Mathematics, 02.08.2019 12:50

English, 02.08.2019 12:50







Mathematics, 02.08.2019 12:50


English, 02.08.2019 12:50

Chemistry, 02.08.2019 12:50

Mathematics, 02.08.2019 12:50

Mathematics, 02.08.2019 12:50

History, 02.08.2019 12:50

Mathematics, 02.08.2019 12:50


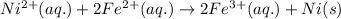
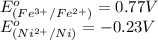
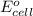 of the reaction, we use the equation:
of the reaction, we use the equation: