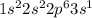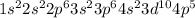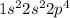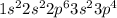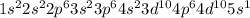Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1


Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
Determine the electron configuration of the following elements. indicate the number of electrons in...
Questions



Mathematics, 03.03.2021 21:20


Mathematics, 03.03.2021 21:20


Business, 03.03.2021 21:20

Advanced Placement (AP), 03.03.2021 21:20

Advanced Placement (AP), 03.03.2021 21:20

Mathematics, 03.03.2021 21:20




Biology, 03.03.2021 21:20





Mathematics, 03.03.2021 21:20

Computers and Technology, 03.03.2021 21:20