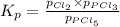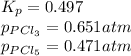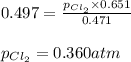Chemistry, 22.08.2019 19:20 wafflewarriormg
The equilibrium constant, kp, for the following reaction is 0.497 at 500k. pcl5(g) pcl3(g) + cl2(g)if an equilibrium mixture of the three gases in a 18.4 l container at 500k contains pcl5 at a pressure of 0.471 atm and pcl3 at a pressure of 0.651 atm, the equilibrium partial pressure of cl2 is atm.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1
You know the right answer?
The equilibrium constant, kp, for the following reaction is 0.497 at 500k. pcl5(g) pcl3(g) + cl2(g)i...
Questions

English, 11.08.2021 22:40

Mathematics, 11.08.2021 22:40

Mathematics, 11.08.2021 22:40



English, 11.08.2021 22:40

Computers and Technology, 11.08.2021 22:40


Mathematics, 11.08.2021 22:40






Biology, 11.08.2021 22:40



Mathematics, 11.08.2021 22:40


Mathematics, 11.08.2021 22:40

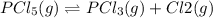
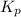 for above reaction follows:
for above reaction follows: