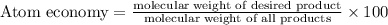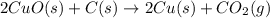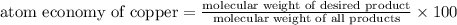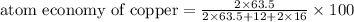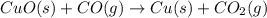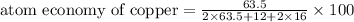Chemistry, 22.08.2019 19:10 GEEKLIFE6598
The following two reactions are possible methods for refining copper in the final step of a smelting process, i. e., getting pure copper (cu) from copper ores found in rocks. calculate the theoretical atom economy for each reaction. a. 2 cuo(s) + c(s) → 2 cu(s) + co2(g) = % b. cuo(s) + co(g) → cu(s) + co2(g) = %

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

You know the right answer?
The following two reactions are possible methods for refining copper in the final step of a smelting...
Questions

Physics, 04.08.2019 09:50

Mathematics, 04.08.2019 09:50



Computers and Technology, 04.08.2019 09:50

History, 04.08.2019 09:50

Chemistry, 04.08.2019 09:50


English, 04.08.2019 09:50






History, 04.08.2019 09:50

Chemistry, 04.08.2019 09:50

Mathematics, 04.08.2019 09:50



History, 04.08.2019 09:50