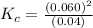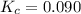Chemistry, 22.08.2019 18:30 bjpvrpow74wq
At high temperatures, carbon reacts with o2 to produce co as follows: c(s) o2(g) 2co(g). when 0.350 mol of o2 and excess carbon were placed in a 5.00-l container and heated, the equilibrium concentration of co was found to be 0.060 m. what is the equilibrium constant, kc, for this reaction?
a. 0.001
b. 0.072
c. 0.090
d. 1.2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

You know the right answer?
At high temperatures, carbon reacts with o2 to produce co as follows: c(s) o2(g) 2co(g). when 0.350...
Questions





Computers and Technology, 16.02.2021 05:10


Arts, 16.02.2021 05:10

English, 16.02.2021 05:10

English, 16.02.2021 05:10

Mathematics, 16.02.2021 05:10

Mathematics, 16.02.2021 05:10


Spanish, 16.02.2021 05:10

Mathematics, 16.02.2021 05:10


Mathematics, 16.02.2021 05:10


Mathematics, 16.02.2021 05:10

Arts, 16.02.2021 05:10

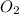 = 0.350 mole
= 0.350 mole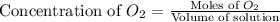
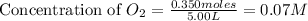
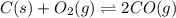
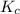 will be,
will be,![K_c=\frac{[CO]^2}{[O_2]}](/tpl/images/0188/5908/f4b67.png)
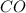 at equilibrium is, 0.060 M
at equilibrium is, 0.060 M