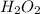Chemistry, 22.08.2019 18:10 ggdvj9gggsc
Given the reaction: h2o2(l) ⇌ h2(g) + o2(g) the forward reaction is endothermic. determine which of the following changes would result in equilibrium shifting towards the products. i. increase h2 ii. decrease o2 iii. add a catalyst iv. increase the temperature v. increase h2o2

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

You know the right answer?
Given the reaction: h2o2(l) ⇌ h2(g) + o2(g) the forward reaction is endothermic. determine which of...
Questions


Mathematics, 20.04.2020 19:21




World Languages, 20.04.2020 19:21



Mathematics, 20.04.2020 19:21



History, 20.04.2020 19:21



Social Studies, 20.04.2020 19:21



Computers and Technology, 20.04.2020 19:21


English, 20.04.2020 19:21

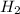 and
and 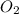 and reactant is
and reactant is