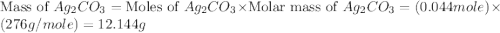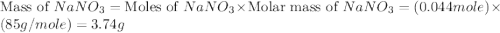Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 4.25 g of sodium carbonate is mixed with one containing 7.50 g of silver nitrate. how many grams of each of the following compounds are present after the reaction is complete?
i) sodium carbonate
ii) silver nitrate
iii) silver carbonate
iv) sodium nitrate

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 23.06.2019 09:00
A0.10 m aqueous solution of sodium sulfate is a better conductor of electricity than a 0.10 m aqueous solution of sodium chloride. which of the following best explains this observation? (a) sodium sulfate is more soluble in water than sodium chloride. (b) sodium sulfate has a higher molar mass than sodium chloride. (c) to prepare a given volume of 0.10 m solution, the mass of sodium sulfate needed is more than twice the mass of sodium chloride needed. (d) more moles of ions are present in a given volume of 0.10 m sodium sulfate than in the same volume of 0.10 m sodium chloride. (e) the degree of dissociation of sodium sulfate in solution is significantly greater than that of sodium chloride.
Answers: 2
You know the right answer?
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution...
Questions

Biology, 22.02.2021 01:00




Mathematics, 22.02.2021 01:00

English, 22.02.2021 01:00

Mathematics, 22.02.2021 01:00

Mathematics, 22.02.2021 01:00



Mathematics, 22.02.2021 01:00

English, 22.02.2021 01:00

English, 22.02.2021 01:00

English, 22.02.2021 01:00

Mathematics, 22.02.2021 01:00


Mathematics, 22.02.2021 01:00



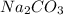 ,
, 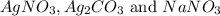 are, 1.908 g, 0 g, 12.144 g and 3.74 g respectively.
are, 1.908 g, 0 g, 12.144 g and 3.74 g respectively.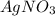 = 7.50 g
= 7.50 g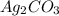 = 276 g/mole
= 276 g/mole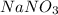 = 85 g/mole
= 85 g/mole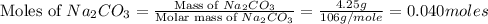
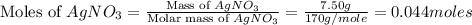
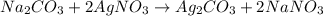
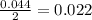 moles of
moles of 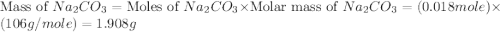
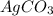 .
.