
Chemistry, 22.08.2019 16:10 achsahjosey
An experiment is carried out to produce hydrogen gas. hydrochloric acid is poured into a glass, and magnesium is added to the acid. the reaction produces a soluble salt and hydrogen gas. 2.1 write a balanced equation for the reaction. 2.2 give the name of the soluble salt. 2.3 has a physical or chemical change taken place? 2.4 2 moles of hydrogen is produced. how many moles of acid reacted?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
You know the right answer?
An experiment is carried out to produce hydrogen gas. hydrochloric acid is poured into a glass, and...
Questions



Mathematics, 03.07.2019 06:30






Mathematics, 03.07.2019 06:30

Physics, 03.07.2019 06:30



History, 03.07.2019 06:30

Mathematics, 03.07.2019 06:30

History, 03.07.2019 06:30




Mathematics, 03.07.2019 06:30

Mathematics, 03.07.2019 06:30

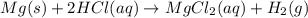
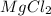
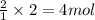 of HCl
of HCl

