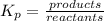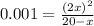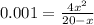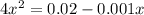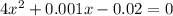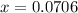Chemistry, 22.08.2019 05:10 chrisraptorofficial
Consider the following gas phase chemical reaction:
a(g) -- > 2b(g)
write down the expression for the equilibrium constant of this reaction.
if the initial concentration of a is 20 atm pressure, the initial concentration of b is 0 atm and the equilibrium constant kp for the reaction is .001 atm-1, calculate the equilibrium concentration of b.
i know the first part of this would be kc = [a] / [b]2 i need the second part

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1

Chemistry, 23.06.2019 23:10
An object was measured by a worker as 14.6cm long, however, the manufacturer specifications list the length of the object at 14.4cm. what is the percent error in the worker's measurement?
Answers: 1

Chemistry, 24.06.2019 01:00
Ethers react with hi to form two cleavage products. one of the products might react further with hi. in the first box below draw the two major products that could be recovered after treatment with one equivalent of hi. in the second box draw the two major products that could be recovered after treatment with excess hi
Answers: 3

Chemistry, 24.06.2019 04:00
How to solve when a mixture of 3-phosphoglycerate and 2-phosphoglycerate is incubated at 25 °c with phosphoglycerate mutase until equilibrium is reached, the final mixture contains six times as much 2- phosphoglycerate as 3-phosphoglycerate. which one of the following statements is most nearly correct, when applied to the reaction as written? (r = 8.315 j/mol·k; t = 298 k)
Answers: 1
You know the right answer?
Consider the following gas phase chemical reaction:
a(g) -- > 2b(g)
write down the...
a(g) -- > 2b(g)
write down the...
Questions

Mathematics, 13.12.2021 23:20

Mathematics, 13.12.2021 23:20


Mathematics, 13.12.2021 23:20



Mathematics, 13.12.2021 23:20

History, 13.12.2021 23:20

Computers and Technology, 13.12.2021 23:20

History, 13.12.2021 23:20

English, 13.12.2021 23:20





Biology, 13.12.2021 23:20

English, 13.12.2021 23:20

SAT, 13.12.2021 23:20


SAT, 13.12.2021 23:20

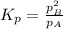 and equilibrium concentration of B is 0.141 atm.
and equilibrium concentration of B is 0.141 atm.