
The solubility of water in diethyl ether has been reported to be 1.468 % by mass.' assuming that 23.0 ml of diethyl ether were allowed to become saturated with water before used and that the 1.2 g magnesium was the limiting reagent, what percentage of the product is expected to be lost due to the water in the solvent if the ether is not anhydrous?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3
You know the right answer?
The solubility of water in diethyl ether has been reported to be 1.468 % by mass.' assuming that 23....
Questions


Mathematics, 27.10.2020 01:00

English, 27.10.2020 01:00

Spanish, 27.10.2020 01:00







Mathematics, 27.10.2020 01:00



Mathematics, 27.10.2020 01:00

Mathematics, 27.10.2020 01:00




Chemistry, 27.10.2020 01:00

Mathematics, 27.10.2020 01:00


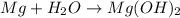
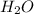 = 18 g
= 18 g


