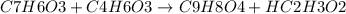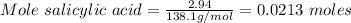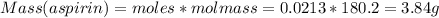Chemistry, 21.08.2019 22:30 hooplikeapro
What is the theoretical yield of aspirin (180.2 g/mol) in the reaction of 2.94 grams of salicylic acid (138.1 g/mol) with excess acetic anhydride (102.1 g/mol)? show your work. include units and round your final answer appropriately.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
You know the right answer?
What is the theoretical yield of aspirin (180.2 g/mol) in the reaction of 2.94 grams of salicylic ac...
Questions



Mathematics, 20.04.2020 19:17


Business, 20.04.2020 19:17









Biology, 20.04.2020 19:17


Chemistry, 20.04.2020 19:17

Social Studies, 20.04.2020 19:17



Mathematics, 20.04.2020 19:18