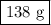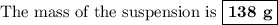Ca + 2h2o --> ca(oh)2 + h2
40g of ca is mixed with 100g of water. calculate mass of soluti...

Chemistry, 21.08.2019 20:30 jescanarias22
Ca + 2h2o --> ca(oh)2 + h2
40g of ca is mixed with 100g of water. calculate mass of solution left after reaction is complete.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 23.06.2019 03:30
Name atleast 3 type of energy associated with the microwave
Answers: 1

Chemistry, 23.06.2019 07:30
Which statement explains which thermometer is more appropriate to measure the temperature of a liquid at 43.6 degrees celsius a) thermometer a, because it measures temperature more accurately than thermometer b b) thermometer b, because it measures temperature more accurately than thermometer a c) thermometer a, because it measures temperature more precisely than thermometer b d) thermometer b, because it measures temperature more precisely than thermometer a
Answers: 2
You know the right answer?
Questions

History, 19.08.2019 13:20


History, 19.08.2019 13:20



Chemistry, 19.08.2019 13:20


Social Studies, 19.08.2019 13:20

Mathematics, 19.08.2019 13:20

Mathematics, 19.08.2019 13:20

Mathematics, 19.08.2019 13:20



Mathematics, 19.08.2019 13:20

Mathematics, 19.08.2019 13:20



Mathematics, 19.08.2019 13:20


Mathematics, 19.08.2019 13:20