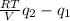Chemistry, 21.08.2019 18:20 allieballey0727
Consider a tank having one inlet molar flowrate qi and one outlet molar flowrate q. it is assumed that the pressure of the gas in the tank is p, the volume of the tank is v and temperature is t. the ideal gas law can be applied. if the volume v and temperature t are constant, find how the pressure varies with time.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3
You know the right answer?
Consider a tank having one inlet molar flowrate qi and one outlet molar flowrate q. it is assumed th...
Questions

German, 28.01.2020 09:31


History, 28.01.2020 09:31




History, 28.01.2020 09:31

History, 28.01.2020 09:31


Mathematics, 28.01.2020 09:31

Mathematics, 28.01.2020 09:31

Mathematics, 28.01.2020 09:31

Mathematics, 28.01.2020 09:31

Mathematics, 28.01.2020 09:31

Mathematics, 28.01.2020 09:31



History, 28.01.2020 09:31


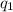
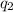
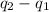 =
= 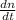 ....... (1)
....... (1)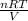
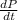 =
= 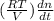
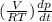
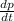 =
=