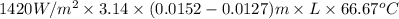Aheat exchanger is to be constructed by forming copper tubing into a coil and placing the latter inside an insulated steel shell. in this exchanger, water will flow inside the tubing, and a hydrocarbon vapor at a rate of 0.126 kg/s will be condensing on the outside surface of the tubing. the inside and outside diameters of the tube are 0.0127 and 0.0152 m, respectively, inlet and exit temperatures for the water are 10 and 32°c, respectively. the heat of conden- sation of the hydrocarbon at a condensing temperature of 88°c is 335 kj/kg, and the heat- transfer coefficient for the condensing vapor is 1420 w/m².k. heat losses from the shell may be neglected. what length of copper tubing will be required to accomplish the desired heat transfer?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

You know the right answer?
Aheat exchanger is to be constructed by forming copper tubing into a coil and placing the latter ins...
Questions





Computers and Technology, 02.11.2019 03:31















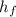 ) = 335 kJ/kg
) = 335 kJ/kg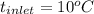 , and
, and 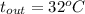
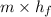
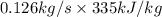
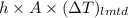
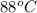 .
.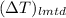 =
= 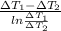
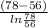
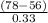
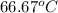
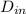 = 0.0127, and
= 0.0127, and 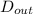 = 0.0152 m
= 0.0152 m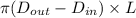 ......... (1)
......... (1)