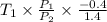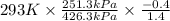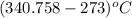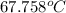Chemistry, 20.08.2019 23:30 kailibug2287
Aclosed tank contains oxygen at 20°c at a gage pressure of 150 kpa. determine the temperature if the oxygen is compressed isentropically to a gage pressure of 325 kpa. the atmospheric pressure is 101.3 kpa and the specific heat ratio of oxygen is 1.40. express your answer in °c to three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

You know the right answer?
Aclosed tank contains oxygen at 20°c at a gage pressure of 150 kpa. determine the temperature if the...
Questions

Mathematics, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

History, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

Social Studies, 10.03.2021 01:00

Computers and Technology, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00


Health, 10.03.2021 01:00


History, 10.03.2021 01:00


English, 10.03.2021 01:00

Biology, 10.03.2021 01:00


Mathematics, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

English, 10.03.2021 01:00


Mathematics, 10.03.2021 01:00

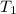 =
= 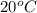 = (20 + 273) K = 293 K
= (20 + 273) K = 293 K 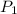 ) will be (150 kPa + 101.3 kPa) = 251.3 kPa
) will be (150 kPa + 101.3 kPa) = 251.3 kPa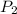 ) will be (325 kPa + 101.3 kPa) = 426.3 kPa
) will be (325 kPa + 101.3 kPa) = 426.3 kPa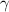 )is given as 1.40.
)is given as 1.40.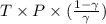 = constant
= constant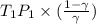 =
= 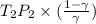
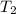 =
=