
Consider the following reaction where kc = 9.52×10-2 at 350 k. ch4(g) + ccl4(g) 2ch2cl2(g)a reaction mixture was found to contain 2.18×10-2 moles of ch4(g), 3.79×10-2 moles of ccl4(g) and 1.09×10-2 moles of ch2cl2(g), in a 1.00 liter container. is the reaction at equilibrium? if not, what direction must it run in order to reach equilibrium? the reaction quotient, qc, equals .the reactiona. must run in the forward direction to reach equilibrium. b. must run in the reverse direction to reach equilibrium. c. is at equilibrium.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2


Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
Consider the following reaction where kc = 9.52×10-2 at 350 k. ch4(g) + ccl4(g) 2ch2cl2(g)a reaction...
Questions

Mathematics, 23.10.2020 21:40

English, 23.10.2020 21:40


Social Studies, 23.10.2020 21:40

Chemistry, 23.10.2020 21:40


Mathematics, 23.10.2020 21:40


Engineering, 23.10.2020 21:50




History, 23.10.2020 21:50

Mathematics, 23.10.2020 21:50



Mathematics, 23.10.2020 21:50

Biology, 23.10.2020 21:50

Mathematics, 23.10.2020 21:50

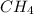 =
= 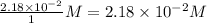
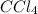 =
= 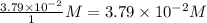
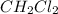 =
= 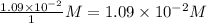
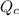 , for this reaction =
, for this reaction = ![\frac{[CH_{2}Cl_{2}]^{2}}{[CH_{4}][CCl_{4}]}](/tpl/images/0183/1287/5dd97.png)
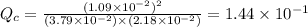
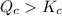 therefore reaction must run in reverse direction to reduce
therefore reaction must run in reverse direction to reduce 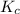 .
.

