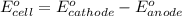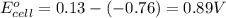Chemistry, 20.08.2019 05:10 strongl3219
Agalvanic (voltaic) cell consists of an electrode composed of zinc in a 1.0 m zinc ion solution and another electrode composed of copper in a 1.0 m copper(ii) ion solution, connected by a salt bridge. calculate the standard potential for this cell at 25 °c. standard reduction potentials can be found here.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2
You know the right answer?
Agalvanic (voltaic) cell consists of an electrode composed of zinc in a 1.0 m zinc ion solution and...
Questions



Health, 25.06.2019 12:00


Mathematics, 25.06.2019 12:00


History, 25.06.2019 12:00


Mathematics, 25.06.2019 12:00




Geography, 25.06.2019 12:00


Geography, 25.06.2019 12:00


Mathematics, 25.06.2019 12:00

English, 25.06.2019 12:00

Geography, 25.06.2019 12:00

Mathematics, 25.06.2019 12:00

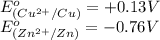
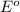 potential will always get reduced and will undergo reduction reaction. Here, copper will undergo reduction reaction will get reduced.
potential will always get reduced and will undergo reduction reaction. Here, copper will undergo reduction reaction will get reduced.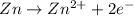
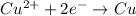
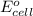 of the reaction, we use the equation:
of the reaction, we use the equation: