

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

You know the right answer?
Classify each of the following substances as either a strong or weak acid, strong or weak base, or a...
Questions





Mathematics, 24.03.2020 17:34





Mathematics, 24.03.2020 17:34






Computers and Technology, 24.03.2020 17:35




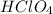 is a strong acid,
is a strong acid, 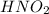 is a weak acid, NaBr is a soluble salt and
is a weak acid, NaBr is a soluble salt and 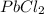 is an insoluble salt
is an insoluble salt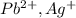 and Hg^{+}[/tex].
and Hg^{+}[/tex].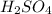 ,
, 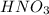 ,
, 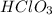 and
and 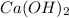 ,
, 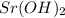 and
and 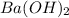 are strong bases.
are strong bases.

