
Chemistry, 19.08.2019 23:20 regalc731ost238
Calculate the mass defect for the formation of phosphorus-31. the mass of a phosphorus-31 nucleus is 30.973765 amu. the masses of a proton and a neutron are 1.00728 and 1.00866 amu, respectively. enter your answer in amu's with five decimal places and no units.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

You know the right answer?
Calculate the mass defect for the formation of phosphorus-31. the mass of a phosphorus-31 nucleus is...
Questions



Mathematics, 01.09.2021 01:00

History, 01.09.2021 01:00


Medicine, 01.09.2021 01:00

Mathematics, 01.09.2021 01:00

Mathematics, 01.09.2021 01:00

English, 01.09.2021 01:00


Mathematics, 01.09.2021 01:00

Mathematics, 01.09.2021 01:00

History, 01.09.2021 01:00


Computers and Technology, 01.09.2021 01:00


Mathematics, 01.09.2021 01:00

Mathematics, 01.09.2021 01:00


![\Delta m=[(n_p\times m_p)+(n_n\times m_n)]-M](/tpl/images/0180/0155/b6e52.png)
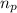 = number of protons
= number of protons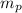 = mass of one proton
= mass of one proton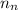 = number of neutrons
= number of neutrons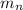 = mass of one neutron
= mass of one neutron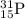
![\Delta m=[(15\times 1.00728)+(16\times 1.00866)]-30.973765\\\\\Delta m=0.27399](/tpl/images/0180/0155/689b8.png)


