
Chemistry, 18.08.2019 04:10 kadence428
The cell potential of the following electrochemical cell depends on the gold concentration in the cathode half-cell: pt(s)|h2(g,1atm)|h+(aq,1.0m)|au3+(a q,? m)|au(s). what is the concentration of au3+ in the solution if ecell is 1.27 v ? express your answer using two significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:50
If a substance is not at its melting or boiling point, as the heat content of a sample of matter increases, its temperature increases the number of intermolecular bonds decreases the space between particles increases the particles move faster
Answers: 2

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
The cell potential of the following electrochemical cell depends on the gold concentration in the ca...
Questions

Mathematics, 28.04.2021 05:00



Mathematics, 28.04.2021 05:00



Mathematics, 28.04.2021 05:00

Biology, 28.04.2021 05:00

Chemistry, 28.04.2021 05:00



Mathematics, 28.04.2021 05:00





Health, 28.04.2021 05:00



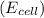 is given as 1.27 V.
is given as 1.27 V.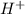 = 1.0 M
= 1.0 M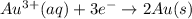
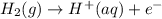
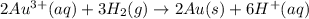
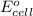 ) for hydrogen is equal to zero.
) for hydrogen is equal to zero.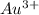 is
is  equal 1.50 V.
equal 1.50 V.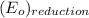 -
- 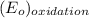
![E_{cell} = E^{o}_{cell} - \frac{0.059}{2}log \frac{[H^{+}]^{6}}{[Au^{3+}]^{2}}](/tpl/images/0175/5466/b4490.png)
![log\frac{1}{[Au^{3+}]^{2}}](/tpl/images/0175/5466/7ec17.png) = 23
= 23![\frac{1}{[Au^{3+}]^{2}}](/tpl/images/0175/5466/cebd5.png) =
= 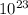
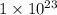
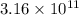
![[Au^{3+}]](/tpl/images/0175/5466/7b483.png) =
= 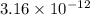 M
M

