
Chloral hydrate (c2h3cl3o2) is a drug formerly used as a sedative and hypnotic.
a. calculate the molar mass of chloral hydrate. b. what amount (moles) of c2h3cl3o2 molecules are in 500.0 g chloral hydrate? c. what is the mass in grams of 2.0 x 10-2 mol chloral hydrate? d. what number of chlorine atoms are in 5.0 g chloral hydrate? e. what mass of chloral hydrate would contain 1.0 g cl? f. what is the mass of exactly 500 molecules of chloral hydrate?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1
You know the right answer?
Chloral hydrate (c2h3cl3o2) is a drug formerly used as a sedative and hypnotic.
a. calculate...
a. calculate...
Questions

History, 08.06.2021 02:00

Mathematics, 08.06.2021 02:00





Mathematics, 08.06.2021 02:00

History, 08.06.2021 02:00


English, 08.06.2021 02:00


Mathematics, 08.06.2021 02:00


Spanish, 08.06.2021 02:00




Mathematics, 08.06.2021 02:00

Mathematics, 08.06.2021 02:00

Mathematics, 08.06.2021 02:00

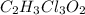 is, 165.5 g/mole
is, 165.5 g/mole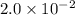 mole chloral hydrate is, 3.31 g
mole chloral hydrate is, 3.31 g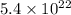

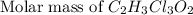 =2(12g/mole)+3(1g/mole)+3(35.5g/mole)+2(16g/mole)=165.5g/mole[/tex]
=2(12g/mole)+3(1g/mole)+3(35.5g/mole)+2(16g/mole)=165.5g/mole[/tex]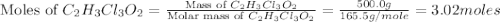
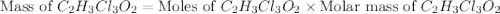
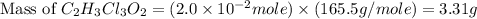
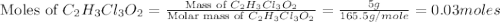
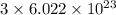 chlorine atoms
chlorine atoms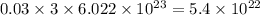 chlorine atoms
chlorine atoms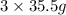 of chlorine present in 165.5 g of
of chlorine present in 165.5 g of 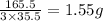 of
of 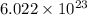 molecules of chloral hydrate has 165.5 g mass of chloral hydrate
molecules of chloral hydrate has 165.5 g mass of chloral hydrate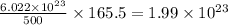 mass of chloral hydrate
mass of chloral hydrate

