
Chemistry, 17.08.2019 19:10 brittanyfox411
A25.00-ml sample of propionic acid, hc3h5o2, of unknown concentration was titrated with 0.141 m koh. the equivalence point was reached when 43.76 ml of base had been added. what is the hydroxide-ion concentration at the equivalence point? ka for propionic acid is 1.3 × 10–5 at 25°c. a. 1.5 × 10-9 m b. 1.1 × 10-3 m c. 1.1 × 10-5 m d. 8.3 × 10-6 m e. 1.0 × 10-7 m

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
A25.00-ml sample of propionic acid, hc3h5o2, of unknown concentration was titrated with 0.141 m koh....
Questions


Social Studies, 09.07.2019 07:30


Mathematics, 09.07.2019 07:30

Mathematics, 09.07.2019 07:30

Biology, 09.07.2019 07:30

Mathematics, 09.07.2019 07:30


Mathematics, 09.07.2019 07:30


Arts, 09.07.2019 07:40


Chemistry, 09.07.2019 07:40

Biology, 09.07.2019 07:40

Biology, 09.07.2019 07:40

Biology, 09.07.2019 07:40


Social Studies, 09.07.2019 07:40


History, 09.07.2019 07:40

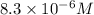
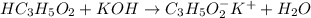
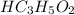 reacts with 1 mol of KOH to produce 1 mol of
reacts with 1 mol of KOH to produce 1 mol of 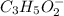
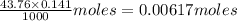
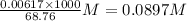
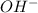 produced at equivalence point is due to hydrolysis of
produced at equivalence point is due to hydrolysis of 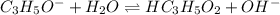
![\frac{[HC_{3}H_{5}O_{2}][OH^{-}]}{[C_{3}H_{5}O_{2}^{-}]}=K_{b}(C_{3}H_{5}O_{2}^{-})=\frac{10^{-14}}{K_{a}(HC_{3}H_{5}O_{2})}](/tpl/images/0175/3912/dc120.png)
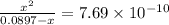
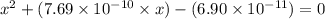
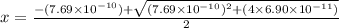 M =
M = 

