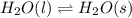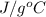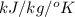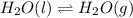Question 2: phase changes (14 points) a. rewrite each of the following equations for phase changes, to include the heat required for the phase change. use values from latent heat and specific heat constant tables when necessary. indicate whether each phase change is endothermic or exothermic. (2 points) i. h2o(l) h2o(s) (1 point) ii. h2o(l) h2o(g) (1 point)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1

Chemistry, 23.06.2019 11:40
Which of the following is true for a reliable scientific source? it cites logic. it cites opinions. it cites valid data. it cites common sense.
Answers: 2
You know the right answer?
Question 2: phase changes (14 points) a. rewrite each of the following equations for phase changes,...
Questions






History, 08.11.2019 18:31

English, 08.11.2019 18:31

History, 08.11.2019 18:31

Mathematics, 08.11.2019 18:31

Mathematics, 08.11.2019 18:31