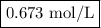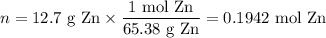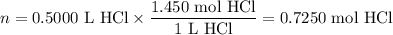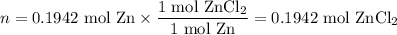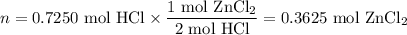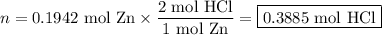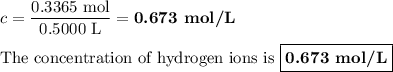Chemistry, 16.08.2019 08:20 JAYDENJONES0111
Zinc dissolves in hydrochloric acid to yield hydrogen gas: zn(s) + 2hcl(aq) --> zncl2(aq) + h2(g) when a 12.7 g chunk of zinc dissolves in 5.00 x 102 ml of 1.450 m hcl, what is the concentration of hydrogen ions remaining in the final solution? 0 m0.388 m0.674 m0.776 m1.06 m

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2


Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
Zinc dissolves in hydrochloric acid to yield hydrogen gas: zn(s) + 2hcl(aq) --> zncl2(aq) + h2(...
Questions


Mathematics, 22.10.2019 00:00


Mathematics, 22.10.2019 00:00

Biology, 22.10.2019 00:00


Social Studies, 22.10.2019 00:00

Biology, 22.10.2019 00:00


Mathematics, 22.10.2019 00:00


English, 22.10.2019 00:00



Mathematics, 22.10.2019 00:00


Biology, 22.10.2019 00:00



Business, 22.10.2019 00:00