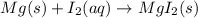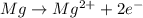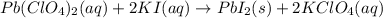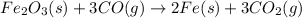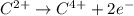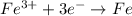Chemistry, 16.08.2019 08:10 avastanleyy
Which of the following chemical equations show(s) oxidation-reduction reactions? 1. mg(s) + i2(aq) --> mgi2(s)2. pb(clo4)2(aq) + 2 ki(aq) --> pbi2(s) + 2 kclo4(aq)3. fe2o3(s) + 3 co(g) --> 2 fe(s) + 3 co2(g)1 only2 only1 and 21 and 32 and 3

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1


Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

You know the right answer?
Which of the following chemical equations show(s) oxidation-reduction reactions? 1. mg(s) + i2(aq) -...
Questions

Mathematics, 14.01.2021 22:30

Physics, 14.01.2021 22:30





Mathematics, 14.01.2021 22:30

English, 14.01.2021 22:30

Physics, 14.01.2021 22:30


Social Studies, 14.01.2021 22:30




Mathematics, 14.01.2021 22:30

Mathematics, 14.01.2021 22:30

Mathematics, 14.01.2021 22:30

Biology, 14.01.2021 22:30