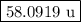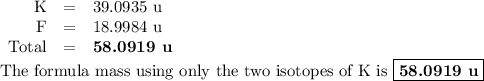Chemistry, 14.08.2019 08:20 petroale000
The two isotopes of potassium with significant abundance in nature are 39k asotopic mass 38.9637 amu, 932.58%) and 41k osotopic mass 40.9618 amu, 6.730%). fluorine has only one naturally occurring isotope, 19f (isotopic mass 18.9984 amu). calculate the formula mass of potassium fluoride.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1
You know the right answer?
The two isotopes of potassium with significant abundance in nature are 39k asotopic mass 38.9637 amu,...
Questions



History, 12.10.2019 14:30

Mathematics, 12.10.2019 14:30



English, 12.10.2019 14:30



Biology, 12.10.2019 14:30

Computers and Technology, 12.10.2019 14:30

Biology, 12.10.2019 14:30

History, 12.10.2019 14:30

Mathematics, 12.10.2019 14:30

Mathematics, 12.10.2019 14:30


English, 12.10.2019 14:30

Biology, 12.10.2019 14:30

Mathematics, 12.10.2019 14:30

Mathematics, 12.10.2019 14:30