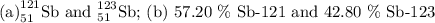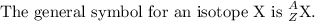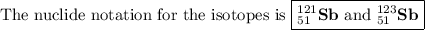Chemistry, 14.08.2019 08:20 sarahkuener
Antimony has many uses, for example, in infrared devices and as part of an alloy in lead storage batteries. the element has two naturally occurring isotopes, one with mass 120.904 amu. the other with mass 122904 amu. (a) write the azx notation for each isotope, (b) use the atomic mass of antimony from the periodic table to calculate the natural abundance of each isotope.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1
You know the right answer?
Antimony has many uses, for example, in infrared devices and as part of an alloy in lead storage bat...
Questions

English, 06.05.2021 03:30


Mathematics, 06.05.2021 03:30

Mathematics, 06.05.2021 03:30

English, 06.05.2021 03:30

Mathematics, 06.05.2021 03:30

Mathematics, 06.05.2021 03:30

Mathematics, 06.05.2021 03:30

Mathematics, 06.05.2021 03:30


English, 06.05.2021 03:30


Mathematics, 06.05.2021 03:30

English, 06.05.2021 03:30



English, 06.05.2021 03:30

Mathematics, 06.05.2021 03:30

English, 06.05.2021 03:30