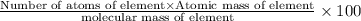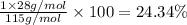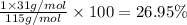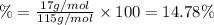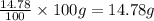Chemistry, 14.08.2019 08:10 anoyinpokep3c3sg
Ammonium dihydrogen phosphate, formed from the reaction of phosphoric acid with ammonia, is used as a crop fertilizer as well as a component of some fire extinguishers, (a) what are the mass percentages of n and p in the compound? (b) how much ammonia is incorporated into 100. g of compound?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2

Chemistry, 23.06.2019 04:20
The graph shows one consequence of urban sprawl. how did urban sprawl contribute to the change in biodiversity
Answers: 2

Chemistry, 23.06.2019 11:30
Distilled water is a completely neutral solution. what is its ph? a. 1 b. 7 c. 14 d. 0
Answers: 2

Chemistry, 23.06.2019 12:10
What is the correct name for hg(no3)2? mercury (i) nitrate mercury (ii) nitrate mercury nitroxide mercury dinitride
Answers: 1
You know the right answer?
Ammonium dihydrogen phosphate, formed from the reaction of phosphoric acid with ammonia, is used as...
Questions


Chemistry, 15.07.2019 09:20


Mathematics, 15.07.2019 09:20


Mathematics, 15.07.2019 09:20

Social Studies, 15.07.2019 09:20



Social Studies, 15.07.2019 09:20


Advanced Placement (AP), 15.07.2019 09:20

Geography, 15.07.2019 09:20



English, 15.07.2019 09:20

Physics, 15.07.2019 09:20


Social Studies, 15.07.2019 09:20

History, 15.07.2019 09:20

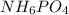 .
.