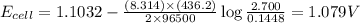Consider the reaction corresponding to a voltaic cell and its standard cell potential. z n ( s ) + c u 2 + ( a q ) ⟶ c u ( s ) + z n 2 + ( a q ) zn(s)+cux2+(aq)⟶cu(s)+znx2+(aq) e o cell = 1.1032 v ecello=1.1032 v what is the cell potential for a cell with a 2.700 m solution of z n 2 + ( a q ) znx2+(aq) and 0.1448 m solution of c u 2 + ( a q ) cux2+(aq) at 436.2 k? c

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1
You know the right answer?
Consider the reaction corresponding to a voltaic cell and its standard cell potential. z n ( s ) + c...
Questions



Mathematics, 29.09.2021 15:10

Mathematics, 29.09.2021 15:10

Mathematics, 29.09.2021 15:10

History, 29.09.2021 15:10


Mathematics, 29.09.2021 15:10

Mathematics, 29.09.2021 15:10

Biology, 29.09.2021 15:10

English, 29.09.2021 15:10


Geography, 29.09.2021 15:10


Social Studies, 29.09.2021 15:10

Mathematics, 29.09.2021 15:10

Mathematics, 29.09.2021 15:10

Mathematics, 29.09.2021 15:10



![E_{cell}=E^o_{cell}-\frac{RT}{nF}\log \frac{[Zn^{2+}]^2}{[Cu^{2+}]}](/tpl/images/0174/9065/fa75b.png)
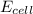 = cell potential of the cell = ?
= cell potential of the cell = ?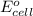 = standard electrode potential = 1.1032 V
= standard electrode potential = 1.1032 V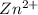 = 2.700 M
= 2.700 M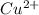 = 0.1448 M
= 0.1448 M