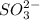Chemistry, 13.08.2019 05:30 naysia2006
Deduce the formulas of the following ionic compounds: (a) iron(lll) sulfide, (b) mercury(ll) nitrate, and (c) potassium sulfite.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
You know the right answer?
Deduce the formulas of the following ionic compounds: (a) iron(lll) sulfide, (b) mercury(ll) nitrat...
Questions

Mathematics, 18.10.2020 02:01

History, 18.10.2020 02:01

Mathematics, 18.10.2020 02:01

Mathematics, 18.10.2020 02:01

History, 18.10.2020 02:01


Mathematics, 18.10.2020 02:01

Mathematics, 18.10.2020 02:01



History, 18.10.2020 02:01

Advanced Placement (AP), 18.10.2020 02:01


History, 18.10.2020 02:01

Mathematics, 18.10.2020 02:01


Mathematics, 18.10.2020 02:01

English, 18.10.2020 02:01


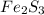
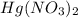
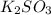
![[Ar]3d^64s^2](/tpl/images/0174/8186/7fd67.png) .
.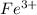 ion, this element will loose 3 electrons.
ion, this element will loose 3 electrons.![[Ne]3s^23p^4](/tpl/images/0174/8186/9a0fd.png) .
.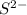 ion, this element will gain 2 electrons.
ion, this element will gain 2 electrons. ![[Xe]4f^{14}5d^{10}6s^2](/tpl/images/0174/8186/13e4d.png) .
.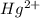 ion, this element will loose 2 electrons.
ion, this element will loose 2 electrons.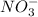
![[Ar]4s^1](/tpl/images/0174/8186/85db5.png) .
.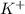 ion, this element will loose 1 electron.
ion, this element will loose 1 electron.