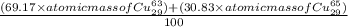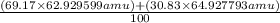Chemistry, 13.08.2019 05:20 johnsonkia873
The atomic masses of the two stable isotopes of copper 63cu29 (69.17 percent) and 65cu29 (30.83 percent)â are 62.929599 and 64.927793 amu, respectively. calculate the average atomic mass of copper.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1
You know the right answer?
The atomic masses of the two stable isotopes of copper 63cu29 (69.17 percent) and 65cu29 (30.83 perc...
Questions



Mathematics, 03.02.2020 17:47



English, 03.02.2020 17:47


Mathematics, 03.02.2020 17:47

Mathematics, 03.02.2020 17:47

Mathematics, 03.02.2020 17:47

Mathematics, 03.02.2020 17:47


Social Studies, 03.02.2020 17:47


Mathematics, 03.02.2020 17:47

History, 03.02.2020 17:47

Business, 03.02.2020 17:47

Mathematics, 03.02.2020 17:47

French, 03.02.2020 17:47

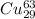 atoms and 30.83 number of
atoms and 30.83 number of 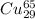 atoms.
atoms.