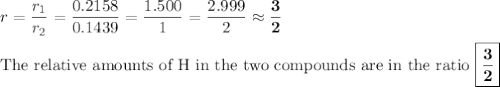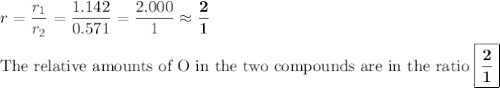Chemistry, 13.08.2019 05:20 quanharris2k19
In each case, calculate the appropriate ratio to show that the information given is consistent with the law of multiple proportions. (a) both ammonia (nh3) and hydrazine (n2h4) are composed of nitrogen and hydrogen. ammonia contains 0.2158 g hydrogen for every gram of nitrogen. hydrazine contains 0.1439 g hydrogen for every gram of nitrogen. (b) two of the compounds that consist of nitrogen and oxygen are nitric oxide, also known as nitrogen monoxide (no) and nitrous oxide (n20), which is also known as dinitrogen monoxide. nitric oxide contains 1.142 g oxygen for every gram of nitrogen. nitrous oxide contains 0.571 g oxygen for every gram of nitrogen.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1
You know the right answer?
In each case, calculate the appropriate ratio to show that the information given is consistent with...
Questions



Mathematics, 15.07.2019 05:40








Mathematics, 15.07.2019 05:40

Mathematics, 15.07.2019 05:40


Biology, 15.07.2019 05:40


Spanish, 15.07.2019 05:40

Biology, 15.07.2019 05:40



Mathematics, 15.07.2019 05:40