
Chemistry, 13.08.2019 05:10 natajaeecarr
Below is a proposed mechanism for the decomposition of h2o2. h2o2 + i– → h2o + io– slow h2o2 + io– → h2o + o2 + i– fast which of the following statements is incorrect? a. io– is a catalyst. b. the reaction is first-order with respect to [i–]. c. the reaction is first-order with respect to [h2o2]. d. the net reaction is 2h2o2 → 2h2o + o2. e. i– is a catalyst.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3


Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1
You know the right answer?
Below is a proposed mechanism for the decomposition of h2o2. h2o2 + i– → h2o + io– slow h2o2 + io– →...
Questions

History, 26.06.2019 06:00


Physics, 26.06.2019 06:00

Mathematics, 26.06.2019 06:00


Mathematics, 26.06.2019 06:00

English, 26.06.2019 06:00




English, 26.06.2019 06:00

Biology, 26.06.2019 06:00

Social Studies, 26.06.2019 06:00

Social Studies, 26.06.2019 06:00

English, 26.06.2019 06:00


Mathematics, 26.06.2019 06:00

Geography, 26.06.2019 06:00

Physics, 26.06.2019 06:00

English, 26.06.2019 06:00

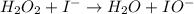 (slow)
(slow)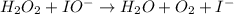
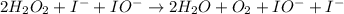
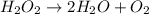
![k[H_{2}O_{2}][I^{-}]](/tpl/images/0174/7990/a32b4.png)
![[I^{-}]](/tpl/images/0174/7990/13772.png) and it is also first order reaction with respect to
and it is also first order reaction with respect to ![[H_{2}O_{2}]](/tpl/images/0174/7990/955b6.png) .
.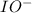 is a catalyst.
is a catalyst.

