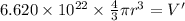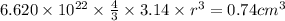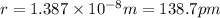Chemistry, 13.08.2019 05:10 gachaperson123
Acube made of platinum (pt) has an edge length of 1.0 cm. (a) calculate the number of pt atoms in the cube. (b) atoms are spherical in shape. therefore, the pt atoms in the cube cannot fill all the available space. if only 74 percent of the space inside the cube is taken up by pt atoms, calculate the radius in picometers of a pt atom. the density pt is 21.45 g/cm3, and the mass of a single pt atom is 3.240 x 10^-22 g. (the volume of a sphere of radius r is 4/5ïr^3).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1
You know the right answer?
Acube made of platinum (pt) has an edge length of 1.0 cm. (a) calculate the number of pt atoms in th...
Questions


Mathematics, 17.07.2019 01:30


Mathematics, 17.07.2019 01:30

Biology, 17.07.2019 01:30









English, 17.07.2019 01:30

Physics, 17.07.2019 01:30




History, 17.07.2019 01:30

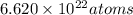 .
.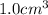
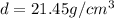
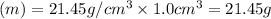
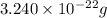
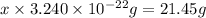
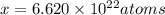
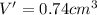
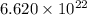 atoms is
atoms is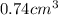 .
.