
Chemistry, 13.08.2019 04:30 emmaguentherp3hjd3
The oxidation of ammonia produces nitrogen and water via the following reaction: 4nh3(g) + 3o2(g) → 2n2(g) + 6h2o(l) suppose the rate of formation of h2o(l) is 3.0 mol/(l ∙ s). which of the following statements is true? a. the rate of consumption of nh3 is 0.50 mol/(l ∙ s). b. the rate of consumption of nh3 is 2.0 mol/(l ∙ s). c. the rate of formation of n2 is 2.0 mol/(l ∙ s). d. the rate of formation of n2 is 1.3 mol/(l ∙ s). e. the rate of consumption of o2 is 2.0 mol/(l ∙ s).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 23.06.2019 06:30
Achemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 no(g) + cl2(g) < => 2 nocl(g) kp = 2 x 10^(-6)he fills a reaction vessel at this temperature with 13. atm of nitrogen monoxide gas and 12. atm of chlorine gas. use this data to answer the questions: a. can you predict the equilibrium pressure of noci, using only the tools available to you within aleks? y/nb. if you said yes, then enter the equilibrium pressure of nocl at right. round your answer to 1 significant digit.
Answers: 1

Chemistry, 23.06.2019 08:00
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2
You know the right answer?
The oxidation of ammonia produces nitrogen and water via the following reaction: 4nh3(g) + 3o2(g) →...
Questions




History, 22.05.2020 16:57

English, 22.05.2020 16:57


Mathematics, 22.05.2020 16:57

Mathematics, 22.05.2020 16:57

English, 22.05.2020 16:57

English, 22.05.2020 16:57

World Languages, 22.05.2020 16:57



Mathematics, 22.05.2020 16:57





Mathematics, 22.05.2020 16:57

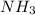 is 2.0 mol/L.s
is 2.0 mol/L.s![-\frac{1}{4}\frac{\Delta [NH_{3}]}{\Delta t}=-\frac{1}{3}\frac{\Delta [O_{2}]}{\Delta t}=\frac{1}{2}\frac{\Delta [N_{2}]}{\Delta t}=\frac{1}{6}\frac{\Delta [H_{2}O]}{\Delta t}](/tpl/images/0174/7635/4514d.png)
![-\frac{\Delta [NH_{3}]}{\Delta t}](/tpl/images/0174/7635/80478.png) represents rate of consumption of
represents rate of consumption of ![-\frac{\Delta [O_{2}]}{\Delta t}](/tpl/images/0174/7635/8d0a6.png) represents rate of consumption of
represents rate of consumption of 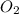 ,
, ![\frac{\Delta [N_{2}]}{\Delta t}](/tpl/images/0174/7635/cd482.png) represents rate of formation of
represents rate of formation of 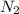 and
and ![\frac{\Delta [H_{2}O]}{\Delta t}](/tpl/images/0174/7635/668d2.png) represents rate of formation of
represents rate of formation of 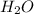 .
.![-\frac{1}{4}\frac{\Delta [NH_{3}]}{\Delta t}=\frac{1}{6}\frac{\Delta [H_{2}O]}{\Delta t}](/tpl/images/0174/7635/898d9.png)
![\frac{\Delta [H_{2}O]}{\Delta t}=3.0 mol/(L.s))](/tpl/images/0174/7635/830f4.png)
![-\frac{\Delta [NH_{3}]}{\Delta t}=\frac{4}{6}\frac{\Delta [H_{2}O]}{\Delta t}](/tpl/images/0174/7635/d2499.png)
![-\frac{\Delta [NH_{3}]}{\Delta t}=\frac{4}{6}\times 3.0 mol/(L.s)=2.0 mol/(L.s)](/tpl/images/0174/7635/32306.png)


