
Which of the following oxidizing agents will oxidize br– but not cl–? 2br– ⇔ br2(l) + 2e– e°ox = –1.065 v 2cl– ⇔ cl2(g) + 2e– e°ox = –1.359 v cr2o72–(aq) + 14h+(aq) + 6e– t h i s a n s w e r i s s e t a s c o r r e c t 7h2o(l) + 2cr3+(aq) e°red =+1.33 v mno4–(aq) + 8h+(aq) + 5e– ⇔ mn2+(aq) + 4h2o(l) e°red = +1.51v no3–(aq) + 4h+(aq) + 3e– ⇔ no(g) + 2h2o(l) e°red =+0.96 v

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1

Chemistry, 23.06.2019 02:00
The plant food contains nh4)3po4 what tests would you run to verify the presence of the nh4 ion and the po4 ion
Answers: 2

Chemistry, 23.06.2019 03:40
Write the overall equation for the reaction occurring in lithium battery?
Answers: 3
You know the right answer?
Which of the following oxidizing agents will oxidize br– but not cl–? 2br– ⇔ br2(l) + 2e– e°ox = –1...
Questions

Mathematics, 17.12.2020 23:20

Mathematics, 17.12.2020 23:20



Mathematics, 17.12.2020 23:20

History, 17.12.2020 23:20



English, 17.12.2020 23:20

Mathematics, 17.12.2020 23:20

Mathematics, 17.12.2020 23:20




English, 17.12.2020 23:20


Mathematics, 17.12.2020 23:20

Law, 17.12.2020 23:20


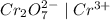 system oxidizes
system oxidizes 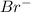 but not
but not 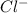 .
.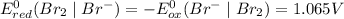
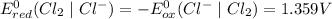
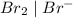 system but have lesser standard reduction potential than
system but have lesser standard reduction potential than 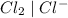 system will oxidize
system will oxidize 


