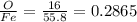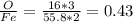Chemistry, 13.08.2019 01:30 katwright1124
Both feo and fe2o3 contain only iron and oxygen. the mass ratio of oxygen to iron for each compound is given in the following table:
compound mass o : mass fe
feo 0.2865
fe2o3 0.4297
show that these data are consistent with the law of multiple proportions.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aspirin has a density of 1.40 g/cm3 what is the volume in cubic centimeters of a tablet weighing 320 mg ?
Answers: 1

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1
You know the right answer?
Both feo and fe2o3 contain only iron and oxygen. the mass ratio of oxygen to iron for each compound...
Questions


Geography, 29.01.2020 22:54

English, 29.01.2020 22:54

English, 29.01.2020 22:54

Chemistry, 29.01.2020 22:54


Biology, 29.01.2020 22:54




Social Studies, 29.01.2020 22:55

Biology, 29.01.2020 22:55



Mathematics, 29.01.2020 22:55

Mathematics, 29.01.2020 22:55


Spanish, 29.01.2020 22:55

History, 29.01.2020 22:55